Abstract
Introduction:
ITP is an acquired autoimmune disorder characterized by isolated thrombocytopenia and an increased risk of bleeding. Paradoxically, ITP is also associated with an increased risk of thrombosis, which may be exacerbated with TPO-RA-treatment. The underlying mechanism(s) involved in the development of thrombosis in ITP and especially in TPO-RA-treated ITP-patients remain poorly understood.
MVs released from activated/apoptotic cells are procoagulant due to the presence of tissue factor (TF) and phospholipids such as phosphatidylserine (PS) on their membrane. MVs have been shown to be increased in ITP-patients but the prothrombotic role of these MVs before and after treatment with TPO-RA is unclear. We measured MV-associated thrombin generation, PS-dependent thrombin generation in plasma, TF-activity and PS-activity in plasma of ITP-patients vs controls and investigated the effect of TPO-RA on these measurements.
Methods:
In 15 controls and 11 ITP patients, before TPO-RA and 2 and 6 weeks after the initiation of TPO-RA, citrated plasma was prepared (2000gx20 min) and immediately frozen. After thawing of plasma, MVs were isolated by centrifugation (17,000gx30 min), the supernatant removed and the remaining MV-pellet washed twice. Isolated plasma-derived MVs were added to pooled normal plasma (PNP), and to obtain measureable thrombin generation, antibodies against tissue factor pathway inhibitor were also added to the PNP. The ability of MVs to generate thrombin was measured by the calibrated automated thrombogram method and the thrombin generation parameters lag time (LT), peak, endogenous thrombin potential (ETP), time to peak (ttPeak) and velocity index (VI) were calculated by the Thrombinoscope software.
To estimate the contribution of procoagulant PS, PS-activity in plasma (PS equivalents) was measured with the Zymuphen MP-activity assay. In addition, thrombin generation was measured directly in plasma where only TF (1pM), but not PS, had been added (PS-dependent thrombin generation). TF-activity in plasma was measured with the Zymuphen MP-TF-activity assay.
Friedman test with Dunn's multiple comparisons was used to compare measurements in ITP-patients before and after TPO-RA-treatment. Kruskal-Wallis test was used to compare measurements in ITP-patients and controls.
Results:
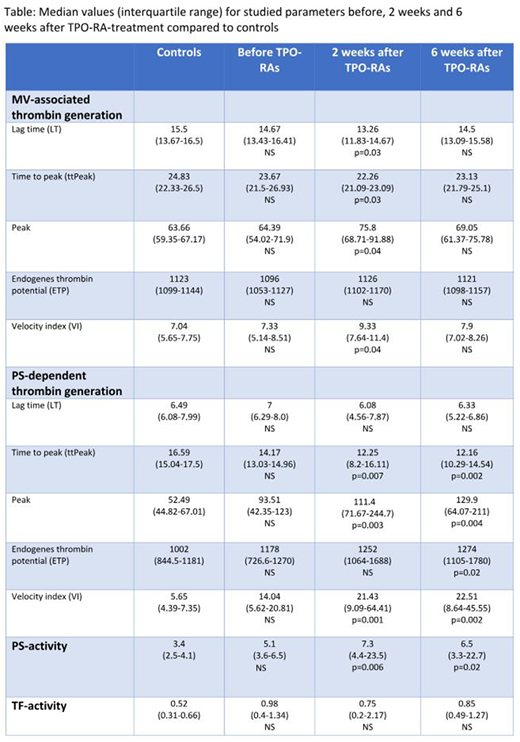
Median age of ITP-patients and controls: 53 and 50 years. Eight (73%) were on romiplostim and three (27%) were on eltrombopag. Median values (IQR) for all measurements before, 2 weeks and 6 weeks on TPO-RA-treatment and in controls are shown in the table.
ITP-patients before treatment with TPO-RAs vs controls: No significant difference was found in MV-associated thrombin generation, PS-activity or TF-activity in plasma. There was a trend towards a higher peak, VI and ETP and lower ttPeak in PS-dependent thrombin generation in plasma of ITP-patients vs controls; lack of statistical significance may be due to the small sample size.
TPO-RA-treated ITP-patients (2 and 6 weeks) vs controls: MV-associated thrombin generation: significant changes after 2 weeks (shorter LT/ttPeak (p=0.03/p=0.03), higher peak/VI (p=0.04/p=0.04)). PS-dependent thrombin generation in plasma: significant changes after 2 weeks (shorter ttPeak (p=0.007), higher peak/VI (p=0.003/p=0.001)), and after 6 weeks (shorter ttPeak (p=0.002), higher peak/ETP/VI (p=0.004/p=0.02/p=0.002)). PS-activity in plasma: significant changes after 2 and 6 weeks (p=0.006/p=0.02). TF-activity in plasma: no significant changes.
ITP-patients before vs after TPO-RA-treatment (2 and 6 weeks): Only MV-associated thrombin generation was significantly increased after 2 weeks (higher peak/VI (p=0.03/p=0.04)).
Conclusions: Compared with controls, treatment with TPO-RAs increases PS-dependent thrombin generation and PS-activity in plasma that is partly accompanied by an increase in MV-associated thrombin generation, but not with an increase in TF-activity in plasma. Similarly, we find a trend of increase in PS-dependent thrombin generation in ITP vs controls. This suggests that PS-positive MVs, most likely released from activated/apoptotic platelets, may contribute to the pre-existing increased thrombotic risk present in at least some of the patients with ITP, and may be used as a potential marker to estimate the risk of future thromboembolic events in TPO-RA-treated ITP-patients.
Ghanima:Roche, Amgen, Novartis, Bayer, BMS: Other: Personal Fees, Research Funding; GlaxoSmithKline and Pfizer: Other: Personal Fees. Bussel:Prophylix: Consultancy, Research Funding; Protalex: Consultancy; Uptodate: Honoraria; Novartis: Consultancy, Research Funding; Amgen Inc.: Consultancy, Research Funding; Momenta: Consultancy; Rigel: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.